Sanjiv S. Agarwala, MD
Professor of Medicine
Temple University School of Medicine
Chief, Oncology & Hematology
St. Luke’s Cancer Center
Bethlehem, PA
Intralesional therapy (direct therapeutic injection into a lesion) for metastatic melanoma was put on the map by a 1975 story in Cancer1 that reported the case of a 77-year-old male with 64 intracutaneous metastases and a pulmonary metastatic deposit. Over an eight-month period of inoculations with Bacille Calmette-Guérin (BCG), 17/17 of the treated lesions resolved and the pulmonary metastasis regressed more than 50 percent. The report suggested not only locally ablative effectiveness, but induction of host immune anti-tumor activity in regional and distant uninjected metastases through a systemic adjuvant response. Interest receded, however, when anaphylactic reactions and death due to disseminated BCG were reported in a subsequent trial. Randomized trials of BCG also failed to confirm a significant clinical benefit, and this approach ceased to be used in practice.2
Melanoma has remained a major clinical problem, however, and a significant percentage of patients have locally advanced disease at high risk for recurrence, progression, and metastasis despite locoregional therapy such as surgery and radiation. Patients with unresectable, multiple, or locoregionally advanced metastatic Stage IIIB/C or Stage IV M1a melanoma in the subgroup with tumors accessible for direct injection are candidates for new incarnations of intralesional therapy.
Intralesional Therapy Revisited
Further research into intralesional therapy3 has been conducted since the BCG trials, in conjunction with a variety of treatment methods, such as carbon dioxide lasers, cryotherapy, electroporation (ECT), and cytokines (IL-2 and IFN-alpha and beta). Interest has especially been heightened recently by three investigational agents that appear to ablate tumors locally and produce systemic “bystander” effects: Allovectin-7, OncoVEXGM-CSF and PV-10. Given that melanoma is considered to become a systemic disease early in its course, the potential systemic effects of these newer agents could prove important.
Allovectin-7 is a plasmid/lipid complex with the DNA sequences encoding HLA-B7 and ß2 microglobulin, both components of major histocompatibility complex class I (MHC-I). The reduced expression of MHC-I in melanoma cells is thought to enable them to evade recognition by T-cells. Researchers believe that Allovectin-7 will increase the immune system’s ability to recognize and target melanoma cells. The drug induces a fivefold increase in the frequency of HLA-B27 cytotoxic T cells, upregulates/restores MHC-I molecules, and induces a proinflammatory response. In a phase 2 trial of Allovectin-74 including 133 patients with stage IIIB/C and stage IV M1a/b injectable cutaneous, subcutaneous or nodal melanoma lesions, the objective response rate (ORR) was 12 percent. There were no grade 3 or higher toxicities. A phase 3 trial of Allovectin-7 versus dacarbazine (DTIC)/temozolomide in recurrent stage III or IV melanoma with ORR (Complete Response + Partial Response, or CR + PR) at or >24 weeks as the primary endpoint is fully enrolled and awaiting analysis.
OncoVEXGM-CSF is a 2nd generation oncolytic herpes simplex virus encoding GM-CSF. It is thought to replicate only in tumor cells with subsequent lysing of injected tumors. Lysed cells are then taken up by antigen-presenting cells (APCs). There may also be an adaptive antimelanoma response enhanced by local expression of GM-CSF. In a phase 2 trial5 of OncoVEXGM-CSF, 20 percent of patients ultimately achieved a CR and 28 percent achieved an ORR. Ninety-two percent of the responses were durable (lasting at least 6 months), and the majority are ongoing, with a range of 18 to 40 months. Responses, observed in patients with all stages of disease, included complete resolution of visceral deposits.
The phase 36 OPTim trial of OncoVEXGM-CSF has enrolled 360 stage IIIB/IV melanoma patients randomized 2:1 to OncoVEXGM-CSF versus subcutaneous GM-CSF alone. The endpoints are durable response at 6 months and overall survival.
PV-10 contains a small molecule fluorescein derivative. It is a non-pyrogenic solution of Rose Bengal disodium (10% RB) which is not metabolized, has about a 30-minute circulatory half-life and is excreted via bile. While PV-10 is excluded from normal cells, it transits through the plasmalemma (cell membrane) of cancer cells (including liver, breast, and other cancers in addition to melanoma) and accumulates in the lysosomes,7 triggering lysosomal release. It enters cancer cells and not normal cells because the cancer cells have a much higher fluidity (higher lipid content) in their cell membranes than normal cells do; even so, it requires a significant amount of PV-10 to ablate even the cancer cells, which helps explain why no hair loss or stomach lining problems have been observed to date in clinical studies. Autolysis is complete within 30-60 minutes. Acute exposure of antigenic tumor fragments to APCs is believed to produce the “bystander” effect in uninjected tumors. This mechanism is unique in that it leads to immediate reduction in tumor burden concomitant with immunologic activation.
Rose Bengal’s Non-Medical Origins
According to Provectus Pharmaceuticals senior vice president Eric Wachter, PhD, the name Rose Bengal was inspired by its color, which is like that of the deep rose-colored middle-of-the-forehead dot indicating marriage in Bengali and other women in India. A German patent #32584 was granted in Basel, Switzerland on February 1, 1885 to a man named Gnehm8 for a new family of wool dyes combining halogens with fluorescein, and one of them that ultimately included four iodines took the name Rose Bengal. Rose Bengal has been employed as a food dye as well.
The first report of clinical use, in 1914, has Römer adding Rose Bengal to Safranin Victoria Yellow to treat ocular pneumococcal infection.9 Applications as a biological staining agent, mostly ocular,10 but also as an intravenous assay for impaired liver function,11 remained the primary medical use for nearly a century. The “eureka” moment for Rose Bengal as therapy came through this latter function, when a 1980s Japanese test of “Food Red no. 105,” intended to identify possible tumorigenicity, found instead dose-dependent survival increases, and left an unremarkable three-line trace in the literature.12 That trace was destined to have little impact until the late 1990s and the advent of high-powered computer searches. Provectus scientists looking for a laser-activated photodynamic therapy agent with antineoplastic activity identified Rose Bengal as a candidate. After subsequent animal and human study and reformulation, the laser activation aspect proved unnecessary and PV-10 was born.
Encouraging Phase 2 Response Rates
Following promising phase 1 results in 2008,13 we conducted a multicenter, international phase 2 trial14 in 80 patients with measurable Stage III-IV melanoma. Intralesional injections of PV-10 were administered to as many as 10 target and 10 non-target cutaneous, subcutaneous, or nodal lesions. New or incompletely responsive lesions were retreated at weeks 8, 12, or 16, with follow-up to 52 weeks. Target lesions were ³ 0.2 cm diameter, with at least one confirmed per patient by biopsy. Investigators observed up to 1-2 untreated, biopsy-confirmed bystander lesions that were typically small or difficult to access (including visceral lesions). The primary endpoint was objective response rate (ORR) for injected lesions. (Table 1.)
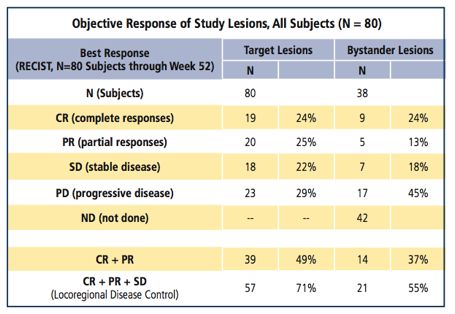
Table 1. Phase 2: Preliminary Efficacy
Among the subjects treated (49 male/31 female, median age 70.0 years [range 33-97]), the median number of PV-10 treatments was 2 (1-4), with a median dose per treatment of 1.6 mL (0.1-15).
Twenty-four percent of patients had complete responses (CR) in target lesions and 25 percent had partial responses (PR) for an ORR of 49 percent. The locoregional disease control (CR+PR+stable disease [SD]) rate was 71 percent. Among the 38 subjects with bystander lesions, CR of their untreated lesions was reported in 24 percent, ORR in 37 percent, and locoregional control in 55 percent. Regression of bystander lesions strongly correlated with response in target lesions.
In a further analysis of the first 40 patients,15 those with CRs achieved significantly longer progression-free survival (11.1 months) than those with stable disease or progressive disease (PD) – 2.8 and 2.7 months, respectively. Responses in injected lesions appeared to be unrelated to disease stage or prior treatment.
No grade 4 or 5 adverse events (AEs) were attributed to PV-10, and over all, AEs were locoregional and predominantly mild-to-moderate.
Coming Next
The planned international phase 3 trial of PV-10 is expected to include up to 300 subjects with stage IIIB-IIIC melanoma. PV-10 will be compared with a control arm of chemotherapy with either dacarbazine (DTIC) or temozolomide, with progression-free survival as a primary endpoint. Enrollment in the 30-month trial is scheduled to begin in the second half of 2012.
The potential for combinations of systemic therapy with intralesional agents is now apparent, and preliminary trials of some intralesional agents in combination with prior therapies have already been initiated.
The bystander effect, which is postulated to occur as a result of an immunologic response to PV-10 and the other intralesional therapies, is especially intriguing. A Phase 2B study is ongoing to examine the immunologic processes whereby PV-10 produces systemic response.
Recently, Provectus announced it had received guidance from the U.S. Food and Drug Administration (FDA) to submit its Phase 3 protocol for review. Provectus is seeking consensus on a design that will qualify for Special Protocol Assessment (SPA) and will support approval of PV-10 for its melanoma indication. The company intends to pursue the SPA path, which would represent an agreement with the FDA that the Phase 3 study design endpoints, statistical analyses, and other components of the planned clinical trials are acceptable to support approval of the product, depending, of course, on the outcomes.
If these intralesional therapies prove to be successful and gain FDA approval, the next logical step would be combination trials with recently approved systemic therapies such as ipilimumab and vemurafenib. Truly, the future for melanoma patients has never been brighter.
References
- Mastrangelo MJ, Bellet RE, Berkelhammer J, et al. Regression of pulmonary metastatic disease associated with intralesional BCG therapy of intracutaneous melanoma metastases. Cancer1975; 36:1305-1308.
- Agarwala SS, Neuberg D, Park Y, Kirkwood JM. Mature results of a phase III randomized trial of bacillus Calmette-Guerin (BCG) versus observation and BCG plus dacarbazine versus BCG in the adjuvant therapy of American Joint Committee on Cancer Stage I-III Melanoma (E1673). Cancer 2004; 100(8):1692-98.
- Testori A, Faries MB, Thompson JF, et al. Local and intralesional therapy of in-transit melanoma metastases. J Surg Oncol 2011 Sep;104(4):391-6. doi: 10.1002/jso.22029.
- Bedikian AY, Richards J, Kharkevitch D, et al. A Phase 2 study of high-dose Allovectin-7 in patients with advanced metastatic melanoma. Melanoma Res 2010 Jun; 20(3):218-26.
- Kaufman HL, Kim DW, DeRaffele G, et al. Local and distant immunity induced by intralesional vaccination with an oncolytic herpes virus encoding GM-CSF in patients with Stage IIIC and IV melanoma. Ann Surg Oncol 2010 Mar;17(3):718-30.
- Kaufman HL, Bines SD. OPTIM Trial: a Phase III trial of an oncolytic herpes virus encoding GM-CSF for unresectable Stage III or IV melanoma. Future Oncol 2010 Jun; 6(6):941-9.
- Wachter E, Dees C, Harkins J, et al. Imaging photosensitizer distribution and pharmacology using multiphoton spectroscopy, in Optical Diagnostics of Living Cells V, Daniel L. Farkas and Robert C. Leif, Editors, Proceedings of SPIE Vol. 4622 (2002) pp. 112-118.
- Gnehm R.Ueber Tetrachlorphtalsäure. Justus Liebigs Annalen der Chemie 1887; 238: 318-338.
- Feenstra RPG and Tseng CG. What is actually stained by rose bengal? Arch Ophthalmol 1992; 110:984-993.
- Sjögren H. (1933) ZurKenntnis der Keratoconjunctivitssicca (Keratitis filiformisbeiHypofunktion der Tränendrüsen). Acta Ophthalmol 1933; 11 suppl. 2:1-151.
- Delprat GD. Studies on liver function: Rose Bengal elimination from the blood as influenced by liver injury. Archives of Internal Medicine 1923; vol. 32:401-410.
- Ito A, Watanabe H, Naito M, Aoyama H, Nakagawa Y, Fujimoto N. Induction of thyroid tumors in (C57BL/6N x C3H/N)F1 mice by oral administration of 9-3′,4′,5′,6′-tetrachloro-o-carboxyphenyl-6-hydroxy-2,4,5,7-tetraiodo-3-isoxanthone sodium (Food Red 105, Rose Bengal B). J Natl Cancer Inst 1986 Jul; 77(1):277-81.
- Thompson JF, Hersey P, Wachter E. Chemoablation of metastatic melanoma using intralesional Rose Bengal. Melanoma Res 2008 Dec; 18(6):405-11.
- Agarwala SS, Thompson JF, Smithers BM, et al. Chemoablation of metastatic melanoma with PV-10.Presented at the Melanoma 2010 Congress. November 4-7, 2010. Sydney, Australia.
- Agarwala SS, Thompson JF, Smithers BM, et al. Chemoablation of metastatic melanoma with Rose Bengal (PV-10). Presented at the American Society of Clinical Oncology 2010 Annual Meeting, June 4-8, 2010, Chicago, IL.