Magnetic resonance imaging (MRI), physician the gold standard of soft tissue imaging, is being used more frequently and in more innovative ways. However, patients with implantable devices are generally ineligible for an MRI. Industry is working to solve the problem and develop MR conditional devices. Experts were questioned at the International Neuromodulation Society (INS) 11th World Congress in Berlin, Germany, June 8-13, 2013, about the status of neurostimulation devices and MRIs.
MRI is being used for a wide array of procedures – from cerebral glioma to MRI-guided gene therapy for brain cancer, heart catheterization, and pericardiocentesis – because it is non invasive and may be more precise than other imaging techniques such as x-rays and computerized tomography (CT) scans, which emit ionizing radiation that accumulates in the body. CT is well-suited for evaluating moderate-to-severe closed head injuries, suspected lung cancer, spine trauma, and suspected appendicitis.
For soft tissue assessment, however, the non-ionizing radiofrequency (RF) signal of MRI is often better suited. For example, in situations in which stroke, seizure, liver lesions, complicated low back pain, or acute joint trauma are known or suspected, MRI would be the preferred choice if safety could be guaranteed, according to MRI safety expert Gregor Schaefers, managing director of MR:comp in Gelsenkirchen, Germany, at a Medtronicsponsored INS symposium.
Lars Jönsson, MD, a neuroradiologist from Sahlgrenska University Hospital in Göteborg, Sweden, speaking at that Medtronic symposium, emphasized that MRI offers the best imaging in many anatomical areas and the only good imaging in some others. MRI also helps patients avoid risky or painful exams, treatment such as myelography, arthroscopy, and endoscopic retrograde cholangiopancreatography (ERCP).
The use of implantable devices – from cardiac pacemakers and implantable cardioverter defibrillators (ICDs) to neurostimulation devices such as spinal cord stimulation (SCS) devices for pain, deep brain stimulation (DBS) devices for seizures, or intrathecal drug delivery systems – also is increasing significantly. There is growing likelihood that a patient with an implant will need an MRI sometime in his or her lifetime, but MRI scanning in patients with devices can potentially cause problems four ways: heating at the lead tip, rotational force (torque) on the device, functional impairment of electronics, and image distortion.
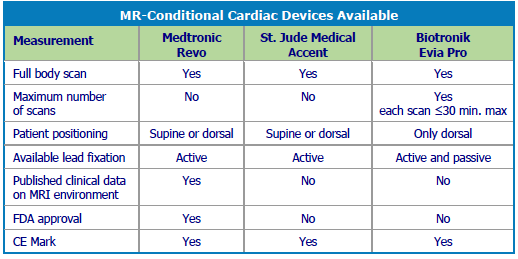
MRI-safe devices are in their very early stages, and Medtronic has the only FDA-cleared MRI-safe pacemaker system. In Europe, several companies have CE Marks for MRI compatible pacing devices. No MRI compatible ICDs have either a CE Mark or FDA clearance. In May 2013, Medtronic received a CE Mark for its CapSureFix Novus 5076 pacemaker lead when paired with an MRI-safe generator.
Other companies also are working to design new devices deemed safe in the MRI environment – referred to as MRconditional devices. However, there is some controversy about whether these new devices are really needed, and some scientists argue that MRIs can safely be performed on patients with existing pacemakers.
Interim findings from the MagnaSafe Registry in 2012 showed no important safety issues with existing devices, but there were some alterations in programming and some transient impedance changes during non-thoracic MRI in patients with pacemakers and ICDs. In 1, check 100 MRIs of patients with 816 pacemakers, 284 ICDs, and 214 leads (with 21% of patients pacemaker-dependent), there were no instances of imagingassociated death, device failure, generator or lead replacement, loss of capture, or electrical reset with MRI, which was performed consistently at 1.5 Tesla (T). That led some experts to conclude that most patients with conventional devices can undergo MRI scans.
GROWING NEED FOR MRI COMPATIBLE DEVICES
According to Medtronic, Germany and the U.S. have the highest rates of MRI procedures, at 97 per 1,000 patients and 91 per 1,000 patients respectively. In 2010, 29 million MRI scans were performed in Western Europe, with 6% of the population scanned each year, and that rate is doubling every 4-5 years. At the same time, >650,000 new pacing devices are implanted globally every year, making it fairly clear that an increasing number of patients with cardiac devices will need an MRI. A 2013 Sorin Group report said that MRI is indicated in 17% of all patients with pacemakers within 12 months of implantation.
Cancer survivors (12 million in the U.S.) are prime candidates for neuromodulation devices, according to Allen Burton, MD, of Houston Pain Associates and former chair of the pain medicine department at MD Anderson Cancer Center. He said that both cancer survivorship and chronic pain attributable to the disease itself or to its treatment are markedly increasing. The lack of MRconditional implantable neurostimulators has largely precluded their use for cancer pain, despite the fact that other treatments may not be as effective.
A common side effect of chemotherapy (especially platinum-based agents, such as the taxanes and vincristine) is peripheral nerve damage, resulting in burning, tingling, and pain in the feet that can be disabling. About a third of long-term cancer survivors have chronic pain, and Dr. Burton estimated that about a third of these have pain that is moderate-to-severe in intensity. Repeated surgeries, other nerve trauma, and poorly controlled radiation are other sources of chronic pain in cancer survivors. Dr. Burton said that the extent to which spinal cord stimulation (SCS) will alleviate that pain is unknown, but for other chronic foot pain patients, neurostimulators implanted in the mid-thoracic spinal cord level work up to 80% of the time. He added that MRI surveillance is used in cancer survivors to monitor for recurrence or spread.
MR:comp’s Schaefers said that among patients with spinal cord stimulators, 70 out of 100 are expected to require an MRI scan over the five-year life of a device, and the pattern is expected to be similar in the U.S. and Canada. A 2010 Medtronic study showed that for patients with implanted devices presenting in an emergency room, 39% got alternative diagnostic imaging, 35% had a spinal cord stimulation device explanted in order to get MRI imaging, and 0.7% of patients with an implanted SCS had an MRI despite contraindications.
The number of 3.0T MRI scanners is likely to increase, mainly for greater diagnostic potential in neurology. MR-conditional pacing systems have been tested at 1.5T, and implanted patients will probably be excluded from more powerful MRI scanners in the future. Furthermore, the MR-conditional pacemakers currently approved by the FDA are only dual-chamber devices. In the future, single-chamber pacemakers could be used even in permanent atrial fibrillation patients who don’t need a dual-chamber device. Thinner and better leads are also expected to result in more widespread and safe use of MRconditional systems.
Real-time MRI surgical procedures also are a coming trend, perhaps with patients no longer having to be awake during DBS because the quality will be so good that surgeons won’t need to talk to their patients. MRI Interventions, for example, recently received a $150,000 grant from the National Institutes of Health’s National Heart, Lung, and Blood Institute (NHLBI) to perform real-time, MRI-guided, catheter-based procedures in the heart.
MRI Risks
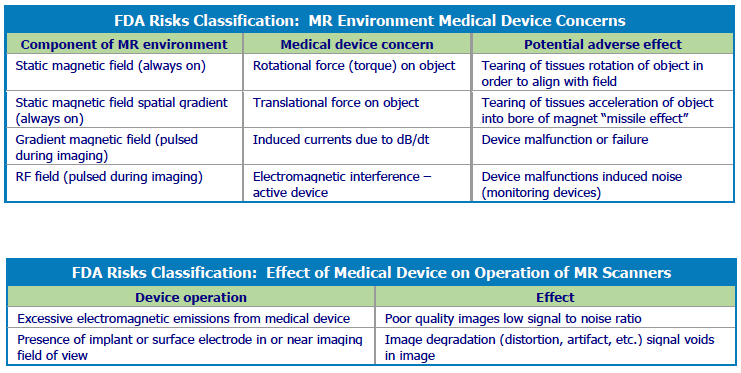
MRI exposure subjects patients to a static magnetic field, gradient magnetic fields, and an RF field. The static magnetic field produced by a 1.5T MRI scanner is 30,000 times stronger than the Earth’s magnetic field, according to safety expert Schaefers. The static magnetic field causes pulling and twisting of ferrous materials within the body and within the environment of the scanner which can cause device damage. In addition, patients may experience vibrations or other potentially disturbing sensations.
Gradient magnetic fields pulsed during imaging sequences can induce voltages on conductive structures with potential for unintentional stimulation of the patient and heating and damage to their device. The pulsed RF field, picked up by unintentionally created antennas in the body, may also create voltages and heating. The potential for RF heating near the electrodes is the primary concern in neurostimulation systems because the electrodes are in brain tissue, next to the spine, or next to peripheral nerves. Both device damage and unintended stimulation of a device are possible.
Current MR-conditional devices are safety rated mostly for 1.5T scanners. However, the use of newer 3.0T scanners is increasing, and they will require a new generation of tests and device adaptations. Steve Manker, program director for Medtronic Neuromodulation MRI-Conditionally Safe Systems, said, “We don’t know yet if the higher frequency will be worse or better. We need to do the testing. On the other hand, the shield technology is not tuned to a specific frequency, so we expect that it will perform quite well at 128 MHz.”
ACHIEVING “MR-CONDITIONAL”
There are three levels of MRI safety:
 MR-safe – This means the device is safe for use in MRI under all conditions. That is, the device is known to be safe or is made out of materials considered safe in the MR environment, such as plastic, silicone or glass, and poses no known hazards in all MR environments.
MR-safe – This means the device is safe for use in MRI under all conditions. That is, the device is known to be safe or is made out of materials considered safe in the MR environment, such as plastic, silicone or glass, and poses no known hazards in all MR environments. MR-conditional – This means the device poses no known hazards in a specified MRI environment with specific conditions of use, such as strength of the magnetic field, the spatial gradient, time varying magnetic fields, radiofrequency fields, and sometimes specific configurations of the device, including routing of neurostimulation system leads. Medtronic has incorporated three display icons into its SCS stimulator: MRI-CS full body scan eligible, MRI-CS head scan eligible, and MRI-CS eligibility cannot be determined.
MR-conditional – This means the device poses no known hazards in a specified MRI environment with specific conditions of use, such as strength of the magnetic field, the spatial gradient, time varying magnetic fields, radiofrequency fields, and sometimes specific configurations of the device, including routing of neurostimulation system leads. Medtronic has incorporated three display icons into its SCS stimulator: MRI-CS full body scan eligible, MRI-CS head scan eligible, and MRI-CS eligibility cannot be determined. MR-unsafe – MR unsafe is broken down into two subtypes – (1) unsafe because of the potential for movement or displacement or (2) unsafe because of the potential for induced currents, excessive heating, or other potential hazards. Manker said that Medtronic uses a shield-based technology for its SureScan SCS leads. While most device leads have a tubular body with four, eight, or 16 conductor wires inside the tube lumen, the SureScan tubular lead body is made up of two urethane layers sandwiching a braided metal layer. He added that implanted patients should be warned about possible harmless sensations of warmth, vibration, or even pulling and tugging during MRI scans.
MR-unsafe – MR unsafe is broken down into two subtypes – (1) unsafe because of the potential for movement or displacement or (2) unsafe because of the potential for induced currents, excessive heating, or other potential hazards. Manker said that Medtronic uses a shield-based technology for its SureScan SCS leads. While most device leads have a tubular body with four, eight, or 16 conductor wires inside the tube lumen, the SureScan tubular lead body is made up of two urethane layers sandwiching a braided metal layer. He added that implanted patients should be warned about possible harmless sensations of warmth, vibration, or even pulling and tugging during MRI scans.
Making a device MR-conditional includes getting heat to dissipate away from vulnerable tissues and components, using techniques such as Faraday cages to keep voltages away from vulnerable components and altering them in order to withstand voltages. Imricor Medical Systems, which makes MRI compatible electrophysiology tools, reportedly has a “special sauce” that it adds to leads in order “to avoid thermal and torsional aspects of risk.”
The Sorin Group report said that the key aspects of validating device compatibility with MRI are:
- Compatibility is linked within a system – device + leads.
- Worst-case conditions for testing to secure clinical
- Measurement certainty.
- Define equipment limitations.
- Diversity of potential adverse effects on the system.
- Organs temperature tolerance.
Manker noted that the first version of ISO/TS 10974:2012(E), a test specification standard that describes the appropriate tests for interactions between MR equipment and active implantable medical devices, was released in May 2012. The Association for the Advancement of Medical Instrumentation (AAMI), a non-profit organization founded in 1967, is working on international MRI safety standards.
If a company doesn’t have the internal expertise, it can turn to testing companies like Schaefers’ MRI:comp or Magnetic Resonance Safety Testing Services (MRSTS) to generate the MRI safety data necessary for regulatory approvals. Clinical trials are not always required, Manker said. The three levels of safety labeling icons and terminology for implant devices were established by the American Society for Testing and Materials (ASTM) International in August 2005. The ‘default’ assumed field strength that these categories apply to is 1.5T.
The three levels of safety labeling icons and terminology for implant devices were established by the American Society for Testing and Materials (ASTM) International in August 2005. The ‘default’ assumed field strength that these categories apply to is 1.5T.
Industry approaches
A 2013 Medscape article written by Luca Santini, MD, PhD, Giovanni Forleo, MD, PhD, and Massimo Santini, MD, said that in order to make its device MR-conditional, Medtronic redesigned the circuit protection normally applied to the power supply, the leads (to minimize and attenuate RF energy discharge at the tip), and the firmware, and the company changed the reed-switch to a Hall sensor.
There are a variety of strategies for circumventing potential lead vulnerabilities.
- Medtronic chose a shield-based technology for its SureScan SCS leads, Manker said. The design incorporates a Faraday cage, an enclosure that blocks external electrical fields in the outer wall of the lead tubing. The Faraday cage keeps the RF energy off the conductor wires and preferentially dissipates heat away from the spinal cord to the much larger surface area of subcutaneous tissue along the length of the lead and safely keeps temperatures low. As mentioned above, Medtronic’s SureScan tubular lead sandwiches a metal layer between two urethane layers.
- Boston Scientific’s Khalid Ishaque, international general manager for neuromodulation (pain and DBS), said that DBS leads must meet higher testing criteria, “In the brain, where there is risk of creating permanent tissue lesions, you can’t generate the heat that you can in the spine where spinal fluid will help to dissipate it.”
Ferrous materials pose a risk with an MRI scanner’s static magnetic field. Manker pointed out that not even Medtronic’s conventional leads contain ferrous materials. He explained that, due to conscious design choices, ferrous materials have been kept to a minimum in their stimulators and in control switches. He also said the stimulator electronics need to be robust to withstand voltages induced by the rapidly pulsing electromagnetic coils that produce the gradient field, and circuitry needs protection from the high radiofrequency energy that could harm the device components.
Current MR-conditional devices are safety rated mostly for 1.5T scanners. However, experts said the growing utilization of newer 3.0T scanners will require a new generation of tests and device adaptations. The 1.5T scanners produce RF energy at 64 MHz, and the 3.0T will produce RF energy at 128 MHz. Manker said, “We don’t know yet if the higher frequency will be worse or better. We need to do the testing. On the other hand, the shield technology is not tuned to a specific frequency, so we expect that it will perform quite well at 128 MHz.”
APPROVED DEVICES
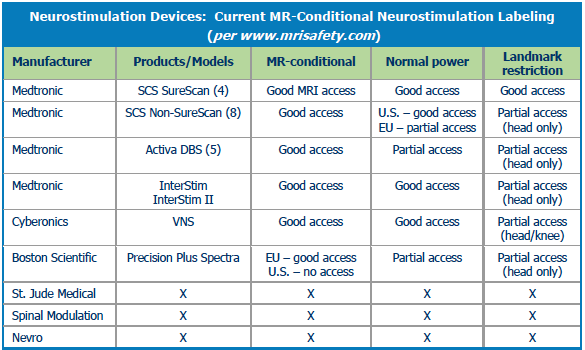
MEDTRONIC
Medtronic has the only MR-conditional device approved for full body scans in the U.S., Canada, and Europe. No implanted neurostimulation devices are MR-safe – yet. MR scanners are rated according to Tesla strength, and current devices are too strong (1.5T) to be used on implanted patients. Manker said, “Since there are 7.0T and 9.0T systems coming, there is no way to guarantee safety in all MR systems.”
Medical devices with MR-safe labeling include some dental implants, shunts, and cosmetic implants, all of which are 100% non-metallic. Medtronic’s full body approval is for 1.5T scanners only. Because no devices can be labeled MR-safe in the neuromodulation market, the term MRI compatible is no longer used.
BOSTON SCIENTIFIC
Boston Scientific has the only neuromodulation device approved for the head in Europe, Canada, and Australia, but it is not cleared in the U.S. Ishaque said that some electrodes or leads will have design changes in order to get clearance for full body scans.
Boston Scientific and MRI Interventions (formerly SurgiVision) have collaborated since 2005 on both neuromodulation technology and implantable cardiac devices. Boston Scientific paid MRI Interventions $13 million in upfront licensing fees, with conditional milestone and royalty payments later.
Ishaque said, “We recognize that a system designed to allow full body MRI under certain conditions can be a valuable option for patients. We have MR-conditional labeling for the head in certain markets. We expect to continue to expand our MRI labeling over the next several years. Although we can’t disclose the exact timing, it is something we continue to work on.”
MRI INTERVENTIONS
The company’s ClearPoint system gives MRI-based stereotactic guidance for neurological procedures; it can be used in the hospital’s existing MRI suite and can be used with both 1.5T and 3.0T scanners. There are about 4,500 1.5T MRI scanners and 550 3.0T MRI scanners in U.S. hospitals. In 2010, the FDA cleared ClearPoint for general neurological procedures. In 2011, the company also got a CE Mark for the system.
MRI Interventions is working with several other companies besides Boston Scientific, including integrating the ClearPoint product line with Brainlab’s iMRI product line, focusing on drug delivery to the brain under MRI guidance.
CYBERONICS
The VNS Therapy System, a vagal nerve stimulator, is rated MR-conditional in the U.S. and in some other markets for imaging of the head and extremities, including knees. The U.S. clearance applies to both 3.0T and 1.5T scanners and is for the treatment of refractory epilepsy and treatment-resistant depression.
Milton Morris, PhD, Cyberonics senior vice president for research and development, said that his company’s device did not need to be modified for MRI safety, “For us, getting the labeling was an exercise in collaboration with the FDA and third parties (the University of Houston) over several years to demonstrate safety under specific conditions for both 1.5T and 3.0T scanners.”
Cyberonics plans to seek clearance for full body scans, but Dr. Morris said that there will have to be modifications in some leads and catheters for that. Cyberonics has a license agreement with Imricor Medical Systems to develop MR-conditional leads for the VNS System. Imricor’s main focus has been development of products enabling MR-guided cardiac ablation procedures, but the company is also working on other MR-conditional implanted devices.
Other
Four other companies in the space do not have approvals for MR-conditional devices but are interested in the area.
- St. Jude Medical. In June 2013 St. Jude made a $40 million equity investment in Spinal Modulation, receiving international distribution rights and an exclusive option to buy the company for up to $300 million. Spinal Modulation’s Axium Neurostimulator System is a neuromodulation device which targets the dorsal root ganglion in order to manage intractable pain in areas hard to treat with traditional SCS systems, including the lower leg, foot, and groin. St. Jude did not respond to repeated requests for information about this device.
- Spinal Modulation.
- Nevro.
- Greatbatch. In the areas of cardiac rhythm management and neuromodulation, Greatbatch has extensive intellectual property critical to enabling MR-conditional capability for implantable devices, according to Christopher Knospe, director of global communications. Greatbatch works with neuromodulation companies to make their products MRconditional. Greatbatch is working with St. Jude but would not discuss other customers/clients.
Through QIG, its subsidiary, Greatbatch is developing its own systems and devices. Greatbatch’s first complete medical device system, Algostim, is a spinal cord stimulation system to treat chronic pain of the trunk and limbs, developed in collaboration with two global leaders in neuromodulation, Richard North, MD, a neurosurgeon at Johns Hopkins University School of Medicine, and Giancarlo Barolat, MD, a Denver neurosurgeon.
Greatbatch is shopping for commercialization partners and plans to ask for FDA clearance and a CE Mark during the second half of 2013, according to Knospe. While the system is not MR-conditional, it was designed with the thought of applying for MR-conditional status down the line.