A Closer Look at Media Messages Versus Research Findings
Walter Alexander
The author is a freelance medical writer living in New York City.
It is generally reassuring when we read health advice that contains a clear message, especially when the advice involves something pleasurable. For instance, eat dark chocolate, and wash it down with red wine (in moderation). But confusion and consternation abound when research produces a mixed message that seems contrary to previous advice. After years of hearing that eating fatty fish or taking fish oil supplements was good for the heart, the eyes, and even mood, the public was puzzled this summer by a study that suggested a risk of prostate cancer in men with high levels of omega-3 fatty acids obtained from these sources.
Although “conclusions” in research are subject to change, in this instance, investigators not connected to the fish oil study complained that the headline-hungry media did not cover all of the facts. They also charged that the headlines were potentially harmful and that the findings were tainted by overreach. A closer look at the study is warranted.
WHAT THE RESEARCH SAID
In a paper published in July,1 Theodore Brasky, PhD, of the Ohio State University Comprehensive Cancer Center in Columbus and a team from the Fred Hutchinson Cancer Research Center in Seattle, Washington, found an association between higher plasma omega-3 fatty acid levels and an increased prostate cancer risk. Looking at data from the Selenium and Vitamin E Cancer Prevention Trial (SELECT) (clinicaltrials.gov Identifier NCT00006392), they measured plasma phospholipid omega-3 levels in 834 men who eventually developed prostate cancer and in 1,393 men who did not.
The men were classified according to their blood levels of three omega-3 fatty acids: eicosapentaenoic acid (EPA), docosapentaenoic acid (DPA), and docosahexaenoic acid (DHA). The researchers found that men in the highest quartile of overall omega-3 levels had, based on prostate cancer severity, an increased risk as follows: from 44% (low-grade cancer: hazard ratio [HR] = 1.44; 95% confidence interval [CI], 1.08–1.93) to 71% (high-grade cancer: HR = 1.71; 95% CI = 1.00–2.94).
Total prostate cancer risk was increased by 43% (HR = 1.43; 95% CI, 1.09–1.88). This finding echoed results from the same author’s 2011 research,2 leading the current Brasky team to conclude that long-chain omega-3 polyunsaturated fatty acids (PUFAs) were involved in prostate tumorigenesis. They said further that recommendations to increase long-chain omega-3 PUFA dietary intake should also address the potential risks.
Brasky et al. also pointed out that the strongest evidence that inflammation plays a role in prostate carcinogenesis is that the prostate cancer precursor lesion, proliferative inflammatory atrophy (an area of highly proliferative but atrophic epithelial cells), displays notable inflammatory infiltrates.(1) The group acknowledged, however, that studies of lifestyle factors associated with reduced inflammation (including the use of aspirin, nonsteroidal anti-inflammatory drugs, and statins) and the intake of long-chain omega-3 PUFAs (EPA, DPA, DHA) have been inconsistent. Obesity is associated with higher inflammation rates, a higher incidence of prostate cancer, and higher death rates. Counter to expectations, their own earlier 2011 study2 had found that:
- high levels of serum phospholipid long-chain omega-3 fatty acids (a biomarker of usual omega-3 fatty acid intake), were associated with a large increase in the risk of high-grade prostate cancer.
- high levels of trans-fatty acids (associated with increased inflammation) were associated with a reduced risk of high-grade prostate cancer.
These findings, the authors write, were a stimulus for the current study,(1) because they raise the possibility that high intakes of omega-3 fatty acids through fish oil supplements, for example, could significantly raise the risk of high-grade prostate cancer. In light of the widespread use of omega-3 fatty acid supplements, the purported health benefits of eating fatty fish, and ongoing clinical trial testing of omega-3 fatty acid supplementation for the prevention of cancer and cardiovascular disease, an investigation of a possible contribution to prostate cancer risk becomes especially important, they said.
WHAT THE MEDIA REPORTED
In an NBC News Health article,3 Dr. Brasky is quoted as stepping somewhat beyond the bounds of the conclusion in the 2013 journal article,1 suggesting that for some men, taking “mega, mega” doses of fish oil supplements is “probably a little bit dangerous.”
The NBC article also notes that whereas the American Heart Association recommends eating fish twice weekly and perhaps taking fish oil capsules for those with heart disease, recent studies have shown that taking extra omega-3 fatty acids has little effect on heart disease.(3) The article also mentions that the researchers did not consider fatty acids in vegetable oils to be related to prostate cancer risk.3
In a study reported in the May 2013 issue of the New England Journal of Medicine,(4) among 12,000 patients with heart disease but without a history of a heart attack, 1 g daily of omega-3 fatty acid supplementation did not reduce morbidity or mortality rates. Therefore, for men hesitant to change course, given that heart disease is much more common than prostate cancer, the shadow of doubt cast on the omega-3’s cardioprotective effects might be a decisive factor.
For William Harris, PhD, Professor of Medicine at University of South Dakota School of Medicine in Vermillion, and a senior scientist at Health Diagnostic Laboratory, Inc., in Richmond, Virginia, decisions triggered by such reports are a real concern. He said in an interview:
Many people with hypertriglyceridemia who are put on prescription fish oils might be tempted into not taking them—and that’s wrong. Also, vastly more individuals are taking fish oil supplements and like to eat high omega-3 fish because they think it’s good for them. The message they are getting now—that omega-3 is in general bad—is incorrect.
In a MedCity News article,5 Dr. Harris emphatically agreed with that statement, charging that although the Brasky study1 certainly had validly added to the prostate cancer evidence base
on omega-3 fatty acids, that team and the media coverage had “extrapolated the findings far beyond the data themselves.”
The unsupportable extrapolation, he said, is “that omega-3 intake causes an increase in prostate cancer risk. … Correlation does not imply causation.”
Dr. Harris pointed out that Brasky et al.(1) provided no data on fish intake or supplement use. So the question of whether fish oil supplements or an intake of more oily fish increases
prostate cancer risk was not tested.
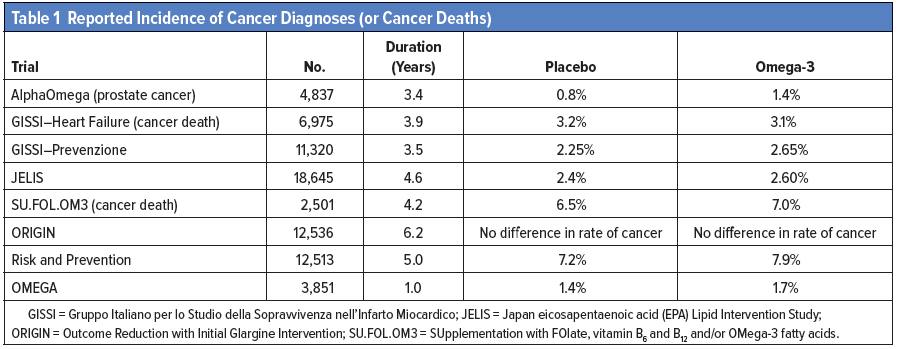
In a further critique of the Brasky article, published on LecturePad,(6) he listed eight major randomized clinical trials (Table 1) of omega-3 fatty acid supplementation that reported incident cancer diagnoses or cancer deaths, with a total enrollment exceeding 78,000 subjects. The list showed small increases in cancer diagnoses or cancer deaths in six of the eight trials, but the increases for omega-3 fatty acid supplementation did not reach statistical significance in any of the trials.
Dr. Harris cited extensive literature on fish intake and higheromega-3 fatty acid intake that demonstrated a lower incidence of prostate cancer incidence and death, better survival among men who already had prostate cancer, and a reduced riskof aggressive prostate cancer. Furthermore, citing World Foundation of Urology data,7 he noted that the incidence of prostate cancer is high in North America and Northern Europe (among Caucasians and African-Americans (63 and 102 per 100,000, respectively) but low in Asia. With the Japanese intake of omega-3 fatty acids at about eight-fold that of Americans and with their blood levels twice as high, one would expect a higher risk. However, the Japanese prostate cancer rate of 22.7 per 100,000 in 2008 was dramatically lower than the U.S. rates of 83.8 per 100,000.(8)
The Brasky article stated that the mean percentage of total omega-3 fatty acids (EPA + DPA + DHA) was 4.66% (range, 4.56%–4.75%) in cancer patients.(1)
“These omega-3 levels,” according to Dr. Harris, “were far lower than would be expected from individuals taking omega-3 supplements.”
The Brasky paper showed significantly lower omega-3 (EPA DPA + DHA) levels in men without prostate cancer at 4.48% (range, 4.41–4.55; P = 0.002).1 The clinical significance of that difference (from 4.48% to 4.66%) was questioned by Richard Deckelbaum, MD, Director of the Institute of Human Nutrition at Columbia University in New York City. He explained in an interview:
I specialize in fatty acid levels, and I was surprised at how small the omega-3 fatty acid differences are, especially for EPA, DPA, and DHA. In our lab, we would not consider these small differences to be biologically significant. Because of the large number of subjects in the SELECT trial, it turned out to be statistically significant, though.
He speculated that perhaps prostate cancer itself could cause these “very small” changes in fatty acid levels. He said, “It’s not quite clear as to where the chicken is and where the egg is.”
Of note, Dr. Harris mentioned the same possibility—that higher levels of omega-3 fatty acids in prostate cancer could be “an innocent bystander.”
Dr. Deckelbaum noted that omega-3 fatty acid intake in North Americans is generally lower than it should be, and he urged that people follow the American Heart Association’s
recommendation to increase omega-3 intake.
Regarding the conclusions drawn from the analyses in Brasky, he said, “I certainly would have been more cautious and would not have made recommendations based on them.”
OMEGA-3 INTAKE VERSUS OMEGA-3 LEVELS
A majority of recent observational studies, said Eliot A. Brinton in an interview, have shown that at least DHA levels— and possibly EPA levels—are associated with certain types of prostate cancer. Dr. Brinton is Director of Atherometabolic Research at Utah Foundation for Biomedical Research and Associate Professor at the University of Utah School of Medicine in Salt Lake City. There does appear to be a difference between EPA and DHA levels in that association, he said.
Brasky et al. reported mean DHA levels (a percentage of the total) of 2.91% in subjects with no cancer and 3.09% in those with high-grade prostate cancer (P = 0.009). For EPA, the mean
levels were 0.61% and 0.65%, respectively (P = 0.40). For DPA they were 0.86% and 0.89%, respectively (P = 0.16). However, Dr. Brinton added that studies of dietary intake of fish oil tend to show an opposite protective relationship with respect to prostate cancer.
“So we are left with an apparent contrast between certain levels of omega-3 and intake of omega-3,” he said.
Dr. Brinton emphasized that no randomized, controlled clinical trial data point one way or the other. The available dietary intake studies, he said, are observational:
You are just asking people what they ate, so these observational studies are just hypothesis-raising—and there we find heterogeneity between EPA and DHA, while the controlled clinical trial data are inconsistent. The best we can tell is that intake is good— and that’s hypothesis-raising—and we only say there’s a lot of confusion regarding what the levels are trying to tell us.
The results of REDUCE-IT (Reduction of Cardiovascular Events with EPA–Intervention Trial) may help to elucidate the issues, Dr. Brinton said. In that ongoing randomized trial, icosapent ethyl (Vascepa, Amarin) capsules, at 4 g/day, or placebo is added to statin therapy in high-risk patients with hypertriglyceridemia (fasting triglyceride levels of 200 to 500 ml/dL) and coronary artery disease (CAD) or CAD risk factors. Vascepa contains only EPA. (Icosapent ethyl is a high-purity prescription form of EPA ethyl ester.) The primary endpoint includes cardiovascular events. REDUCE-IT is being conducted at almost 300 international sites with a target enrollment of about 8,000 patients, with completion anticipated in 6 years. Dr. Brinton is on the trial’s steering committee and has been a speaker and consultant for Amarin.
Although several earlier trials and studies have supported cardioprotection with omega-3 intake, some recent trials of low-dose EPA plus DHA have failed to show that association.
“The ongoing REDUCE-IT trial may resolve whether or not higher doses of pure EPA reliably reduce CVD,” Dr. Brinton said.
His main concern, however, was that the public reporting on the research of the Brasky findings—which clearly did not address fish intake—linked fish intake with prostate cancer.
“They got it all wrong,” he said. The result of these contradictory messages about omega-3 fatty acids, cancer risk, and heart disease? “The public is whipsawed,” he said. Dr. Brinton voiced uneasiness, as well, that the researchers did not adequately highlight some “striking heterogeneity” in their meta-analysis of prospective biomarker studies measuring associations between EPA; DHA; long-chain omega-3 PUFAs; and total, low-grade, and high grade prostate cancer. In each category of cancer type for each of the three omega-3 fatty acids, one or two studies out of four to seven showed positive effects on events—for instance, a relative risk of 0.53 for high-grade cancer with DHA, as stated in the study by Charvarro et al.(9) “I wish they had addressed that,” Dr. Brinton said. He speculated that the puzzling turnaround in cardiovascular outcomes with omega-3 studies might be related to the effective contemporary use of beta blockers, antiplatelet therapy, and statins, making it much more difficult to show benefit from omega-3 fatty acids. Even though these same studies failed to show cardiovascular benefits of fish oil, along with studies showing a possible association between prostate cancer with higher omega-3 levels, Dr. Harris strongly believes that the benefits of a higher intake of omega-3 fatty acids outweigh any possible harm. With the National Vital Statistics report for male prostate cancer deaths at 28,500 in 2010 and 207,500 for ischemic heart disease in the U.S., what he called a “conservative” estimate of a 10% reduction in heart disease death risk stacks up well against a “liberal” assumption of a 50% increase in prostate cancer deaths. Under that model, the chances of dying from ischemic heart disease are still nearly 4.5 times greater that the risk of dying from prostate cancer. “The wealth of data in other prostate cancer studies point in the direction of showing protective effects of omega-3 fatty acids,” he added. Dr. Harris concluded that the study authors and the media alike should discipline themselves to report findings in a balanced context and should “resist the temptation to wildly extrapolate in order to grab headlines”—especially if failing to do so puts patients at risk, he said.
NUMBERS HAVE CONSEQUENCES
The numbers pertaining to prostate cancer remain compelling. The American Cancer Society projects that almost 239,000 new cases of prostate cancer will be diagnosed in 2013 and that nearly 30,000 men will die of this disease. During his lifetime, a man stands a 1 in 6 chance of having prostate cancer.
Dr. Brasky told NBC Health, with regard to his message about fish intake, given the 70% increased risk in high-grade prostate cancer, “I think it’d be a concern for people.”3
Dr. Brinton said, “My bottom line is, ‘keep eating fish.’ ”For Dr. Harris, the critical fact is that omega-3 fatty acids are associated with a reduced risk of the leading cause of death in men and women in the U.S.—cardiovascular disease.
Currently approved omega-3 agents include icosapent ethyl (Vascepa) with EPA only, and omega-3-acid ethyl esters (Lovaza capsules, GlaxoSmithKline), with 47% EPA and 38% DHA. Epanova, Omthera’s investigational drug, contains EPA (range, 50%–60%) and DHA (range, 15%–25%). Phase 3 studies have been completed. AstraZeneca purchased Omthera in May 2013.
REFERENCES
- Brasky TM, Darke AK, Song X, et al. Plasma phospholipid fatty acids and prostate cancer risk in the SELECT trial. J Natl Cancer Inst, July 10, 2013 (online).
- Brasky TM, Till C, White E, et al. Serum phospholipid fatty acids and prostate cancer risk: Results from the prostate cancer prevention trial. Am J Epidemiol 2011;173(12):1429 1439.
- Fox M. Fish oils may raise prostate cancer risks, study confirms. NBC News Health, July 10, 2013. Available at: www.nbcnews. com/health/fish-oils-may-raise-prostate-cancer risks-studyconfirms- 6C10597283. Accessed August 5, 2013.
- The Risk and Prevention Study Collaborative Group. n-3 fatty acids in patients with multiple cardiovascular risk factors. N Engl J Med 2013;368:1800–1808.
- MedCity News. The omega-3/prostate cancer media fiesta:
Mistaking correlation for causation puts patients at risk. July 16, 2013. Available at: http://medcitynews.com/2013/07/theomega- 3prostate-cancer-media-fiesta-mistaking correlationfor- causation-puts-patients-at-risk. Accessed August 5, 2013. - Harris W. Problems with recent study on omega-3 fatty acids and risk for prostate cancer. Available at: www.lecturepad.org/ index.php/commentaries-and-opinions/1153-omega-3-fattyacids- and-risk-for-prostate-cancer. Accessed August 5, 2013.
- World Foundation of Urology. Prostate cancer prevention. Available at: www.prostatecancerprevention.net. Accessed August 15, 2013.
- World Health Organization, International Agency for Research on Cancer. Globocan 2008, Cancer Fact Sheet. Prostate Cancer Incidence, Mortality and Prevalence Worldwide in 2008 Summary. Available at: http://globocan.iarc.fr. Accessed August 7, 2013.
- Chavarro JE, Stampfer MJ, Li H, et al. A prospective study of polyunsaturated fatty acid levels in blood and prostate cancer risk. Cancer Epidemiol Biomarkers Prev 2007;16(7):1364–1370. n