An Integrated Approach for Clinical Trial Patient Retention
Scott H. Connor, Vice President, Marketing, Acurian
 Recruiting the right patients in sufficient numbers to clinical trials is a well-recognized challenge. The growing and equally critical challenge is keeping them in the studies for their duration.
Recruiting the right patients in sufficient numbers to clinical trials is a well-recognized challenge. The growing and equally critical challenge is keeping them in the studies for their duration.
In Phase III trials a full 30% of patients drop out, said Suzanne Elvidge in her article “Importance of Patient- Retention Strategies,” (April 2010, Life Science Leader, citing a CenterWatch analysis). The overall rate might actually be quite worse, according to a study from the Tufts Center for the Study of Drug Development (KI Kaitin, “Growing Protocol Design Complexity Stresses Investigators, Volunteers,” Impact Report, 2008) on 57 Phase II and III protocols. The report showed that between assessments in 1999- 2002 and in 2003-2006 clinical trial retention rates fell from 69% to 48%. Such high dropout rates can endanger the statistical validity of the clinical trial data analyses and render the findings unacceptable to worldwide regulatory agencies. Attempting to rebuild a depleted patient cohort with replacements midstream may be prohibitively costly and logistically cumbersome. This makes having patient retention strategies in place proactively an essential ingredient for a successfully conducted clinical trial. Given the clearly growing challenges to ensuring patient retention and research data collection, the modest added program cost of building in such strategies may be easily justified. In fact, many clinical operations directors and trial managers regard patient retention services as an affordable insurance policy to protect against the more severe cost implications caused by lost patients.
Traditional retention programs, however, have done a poor job of establishing success metrics or gaining momentum with research sites because of the very nature of their design. The cottage industry emerging over the last decade focusing on the patient retention portion of the clinical trial equation has attacked the problem in a fragmentary, decentralized manner. The major emphasis has been to reward patient participation with modest gifts, a strategy that is void of tracking or metrics, not to mention scrutiny due to recent changes in industry ethics codes. Further, distribution and warehousing of gifts is often a burden to sites, and ongoing, two-way communications between patients and sites is non-existent in this traditional approach, yet is an important part of patient satisfaction and retention.
New World Order
Acurian (the company), an established leader in patient recruitment and retention, has recently introduced its Retention Manager™ software platform, an innovative, comprehensive program that combines communication software with an array of direct services in support of patient retention. After conducting primary market research with patients, sites and sponsors, the company realized that the patient-site relationship is key to retention, and study coordinators require more efficient methods for forging stronger communication bonds with patients.
Case study data from a well-known telecommunications company in the clinical research space supports the premise that communication technology, such as cell phone text messages, has a positive impact on retention rates. The company compared the retention effects of using text message-based study visit and compliance reminders in a phase II bipolar study, as compared to an identical bipolar study that did not use communication technology. The use of text messaging reduced patients
lost to follow up by 11% and early withdrawal by 9%. Acurian’s Retention Manager goes beyond simple text messaging to include additional technologies and services described later in this paper.
The Conventional Approach
For the past decade, much creativity has been directed towards finding ways to incentivize patients’ ongoing commitment to the clinical trial process. Patients are provided with informational booklets, invitations to join associations, various tchotchkes, thermoses, tote bags and t-shirts with an imprinted study logo, practical health-related aids to exercise or diet (eg, diabetes recipes and food guides), and assorted marketing give-aways—all designed to keep patients mentally engaged in the fact that they have made a commitment to the research project.
Typically, the research coordinator receives a large box packed with the brochures, stuffed animals, tote bags and pedometers, leaving the distribution to the site personnel, and more importantly the tracking of what is being done when to the site coordinator. At the same time, it is universal experience that site coordinators consider themselves to be overworked and ceaselessly “on the firing line.” And with the responsibility to be aware of where perhaps 20 or more patients are in a multi-stage protocol, and in larger centers, juggling multiple patient cohorts in multiple, disparate trials, it is easy to understand how the operant question may be: “Is this putting something else onto my plate or taking something off?”
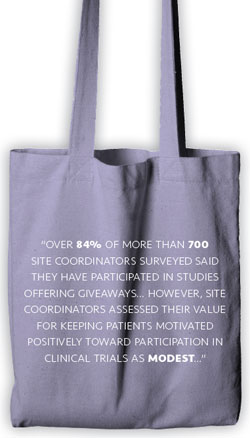
The distribution of various study related gifts is common. Over 84% of more than 700 site coordinators surveyed said they have participated in studies offering giveaways of this nature (Patient Retention Services Assessment. MSP Analytics, Inc. December 2009). However, site coordinators assessed their value for keeping patients motivated positively toward participation in clinical trials as modest (3.1/5 as compared with 4.6/5 for expense reimbursement cards).
It is worth mentioning here the new ethics code of the Pharmaceutical Research and Manufacturers of America (PhRMA) unveiled in July 2008. Its ban on distribution of pens, mugs, pads or other similar items with drug names was unanimously endorsed by member companies, and prominent members (including Eli Lilly & Co., Johnson & Johnson, Pfizer Inc. and GlaxoSmithKline PLC) have issued statements of support. Though enforcement is voluntary, the code has had a chilling effect on distribution of promotional items and other perks throughout the medical community. The questionable value of such tactics for patient retention notwithstanding, this does underscore that in the current climate a stronger emphasis on direct personal and practical support of site managers and patients makes sense.
A further insight into patient motivation, afforded by a study (Clinical Trial Participation Insights, Survey Gizmo, April 2010, sponsored by Acurian) looking at patients who had participated in and remained in clinical trials, is that it was the altruistic aspect, the sense of contributing to the “greater good,” that played the strongest role in patients’ self-assessments of why they had remained enrolled throughout the clinical trial duration. Toward that greater good, the site coordinator’s core obligation lies in seeing that the patient’s portion of the research protocol is carried out. Patients have to make and keep appointments within the stipulated time windows, schedule and submit to lab tests or imaging as required, and stay with the trial throughout the follow-up period. Relentlessly keeping track of patients’ status with regard to all of this is complex and difficult, as is taking appropriate steps when patients miss or fall behind in their appointments. There are associated loose-leaf binders, file drawers and folders stuffed with paperwork, and temperamental software programs to be managed.
Early Warning Signs
Aside from worrying individual patients through the many details of the clinical trial process, the state of the overall data collection project has to be monitored closely. Is a pattern of missed or outside-the-window appointments emerging? Are patients dropping out at an excessive rate? How will the site coordinator identify the problem? Take appropriate corrective actions? Will the sponsor find out that a third of the patients have been lost four months after a problem emerges and at a point too late for reasonable strategic adjustments?
The fundamental insight embodied in the Retention Manager platform is that successfully orchestrating and managing the tasks and details necessary for fulfilling clinical trial protocols while retaining patients in them is an enormous undertaking. When site coordinators are called upon to accomplish the critical synthesis of gathering, communicating and acting on information, on assembling all of the essential pieces unassisted, all too often crucial steps simply get left out. Only a centralized, integrated and structured approach to workflow, one combining effective human services with well-designed software, will do the job.
Reduced Workload
What happens when a patient has not yet made an appointment for a physician visit required by the protocol? The company’s Retention Manager software shows the site coordinator which among the active patients currently eligible to be scheduled for an appointment have not yet made one and therefore need to be focused on urgently. It identifies the patient and, through a set of color-coded warnings, the number of days until the appointment window opens up and until it ends. It also notes if any laboratory testing is needed. Retention Manager generates a constant stream of communication within the system and between the patient and the site coordinator so that each knows what the next step is and what steps have already been taken. While the platform takes on 90% of the work of coordinating and structuring the sequence of tasks to be accomplished, the site coordinator retains the task of ensuring that a particular patient has an appointment within the visit window. The software and Acurian’s retention specialists take up the other tasks, such as informing the patient via calls or text messages about the appointment date, sending reminders about upcoming appointments, noting any requirements (e.g., no eating after midnight for tests), or when there are long intervals between visits, sending general encouragement notes to maintain patients’ interest in and engagement with the clinical trial. The overall upshot is that the site coordinator’s tasks are substantially reduced while simultaneously, general vigilance with respect to the trial’s state of affairs and awareness as to what tasks need to be completed in a particular timeframe are heightened.
This heightened level of communication enables busy study coordinators to maintain a positive bond with patients throughout the entire study, yet requires no significant additional work. The resulting effect is better retention.
Real-time, Actionable Metrics
The availability, at any point during the progress of the trial, of real-time snapshot reports (for example, on patient retention percentages or patient compliance with various aspects of the protocol such as making appointments or scheduling tests) helps site coordinators monitor the trial status and assures sponsors that the project is being competently managed.
When it becomes apparent that patients have not merely missed an appointment or two, but have lost interest and may be considering dropping out, Acurian’s retention specialists engage in an intensive recovery process with direct, respectful personal contacts. The first goal is to identify the underlying causes of patients’ dissatisfaction. Are they unhappy with their physicians? Are travel expenses excessive? Investigators can be switched. Additional travel reimbursement can be arranged. Every effort is made to quickly identify patients’ issues and to ameliorate their concerns and reconnect them with the clinical trial. When the potential effects of a patient dropping out on data analyses, regulatory review, and costs of recruiting and introducing replacements are taken into account, sponsors fully accept that such efforts/expenses are justified.
Instant Gratification
Further practical details such as providing patients with agreed upon reimbursement for travel expenses through the platform’s debit card module are handled seamlessly by the software. For the patient, instant reimbursement for study-related expenses means instant gratification, and validation that study compensation is delivered as advertised. Sites benefit greatly as well. Study coordinators can finally abandon cumbersome and risky payment methods such as live checks. The debit card approach simplifies and secures financial administration, which is typically not a core competency of research professionals. As a result, site staff can focus on enhancing patient care and customer service as part of the retention strategy, rather than handling complaints from patients regarding reimbursement delays or misplaced checks.
The Overall Effect
 What is accomplished through this combination of software and targeted communications? That which is usually a fragmented process, one that at key moments becomes unmanageable by virtue of the sheer volume of necessary tasks, is simplified, prioritized and coordinated—without adding to anyone’s workload, especially the study coordinator. Problems appear “on the radar” with sufficient leadtime for effective intervention so that all those patients who can possibly be reclaimed for the study are brought back in, avoiding compromise to data collection and excessive costs.
What is accomplished through this combination of software and targeted communications? That which is usually a fragmented process, one that at key moments becomes unmanageable by virtue of the sheer volume of necessary tasks, is simplified, prioritized and coordinated—without adding to anyone’s workload, especially the study coordinator. Problems appear “on the radar” with sufficient leadtime for effective intervention so that all those patients who can possibly be reclaimed for the study are brought back in, avoiding compromise to data collection and excessive costs.
A technology and services approach like Retention Manager has a bright future because of what appears to be a gloomy forecast for clinical study design. According to the previously referenced Tufts study, the overall duration of clinical trials has increased 74%, average case report forms have grown from 55 to 180 pages, and consent forms are getting longer and more complicated. Trial protocols have become more complex with higher average numbers of required procedures per protocol. In short, patients are being asked to do more over longer periods of time. This level of commitment must be supported by exceptional site service, vigilant twoway communication, appreciation and support, and instant reimbursement. Without this level of insurance policy in place, trial managers are risking their trial outcomes in an environment that is ultra competitive and unforgiving.
 About Acurian
About Acurian
Acurian is a leading full-service provider of clinical trial patient recruitment and retention solutions for the life sciences industry. Through its proprietary patient panel of over 65 million patients, centralized advertising capabilities, and a fully hosted enrollment management technology platform, Acurian is able to identify, contact, prescreen, and refer patients into clinical trials, all while supporting investigator sites with services to maximize the randomization potential of every referred patient. Since 1998, Acurian has supported over 400 protocols for more than 60 companies. Acurian’s investors include Euclid SR Partners, ProQuest Investments, JP Morgan Partners, Flatiron Partners, CDP Capital Technology Ventures, and Merck Capital Ventures.
For more information about Acurian, visit www.acurian.com
